Not All Lithium Cells Are The Same

Lithium cells and the batteries they are assembled in are available in many chemistries, arrangements, and degrees of quality. Understanding how they differ can determine which one is used in a particular application, the longevity and price of the final battery. In this article, we will discuss how each lithium cell and battery differs from the other by exploring the performance characteristics of lithium batteries, the different types of chemistries, the various construction methods, and the types of available grades.
Lithium Battery Basics
Let’s start from the beginning. How does a battery create energy? For a battery to create energy, an electrochemical reaction occurs between a positively charged material (cathode), a negatively charged material (anode) and an electrolyte in the form of a liquid, gel or solid substance. This electrochemical reaction creates a flow of electrons between the electrodes, thus creating energy.
In the case of a Sealed Lead Acid (SLA) battery, this is between a Lead Oxide Plate (cathode), Lead Plate (anode) and a Sulfuric Acid Water mix (Electrolyte). These are designed into 2.1V cells and series to create a 12.6V battery. Your standard car battery.
A Lithium battery still utilises a cathode, anode and electrolyte, but because Lithium is much smaller and lighter, a lot of Lithium can be stored in both electrodes. This creates a higher energy density that produces a higher voltage. A single lithium-ion cell can produce a voltage of 3.6 volts or higher, depending on the cathode materials.
In the case of a Lithium Iron Phosphate (LiFepo4) battery, the anode cell is made of graphite, the electrolyte, a solution of lithium salts in a mixture of specially devised solvents. Where a LifePO4 battery really comes into its own is the cathode. Where a LifePO4 battery gets its name from. This cell consists of negatively charged phosphate irons bonded with positively charged iron ions. This creates a tightly bonded structure that has high chemical stability and density.
This results in a battery that has a low self-discharge rate, quick recharge time, flat discharge curve capable of delivering a high amount of cycles in a battery that is less than half the weight of an equivalent SLA battery. A LifePO4 battery can also be designed to produce a similar voltage to a SLA battery and be configured to fit into standard SLA cases for ease of replacement.
However, the SLA battery does have some advantages over Lithium, and although Lithium is a great SLA replacement, it is not suitable for every application. These are usually applications requiring extremely high discharge rate, exposure to persistent extreme heat, or price sensitivity. In these cases, an SLA battery may still be preferred.
But with technology continuing to advance in both lithium batteries and their applications, the choice between which one to use is not always clear and requires more profound analysis, more so in industrial applications.
Different Lithium Battery Chemistries
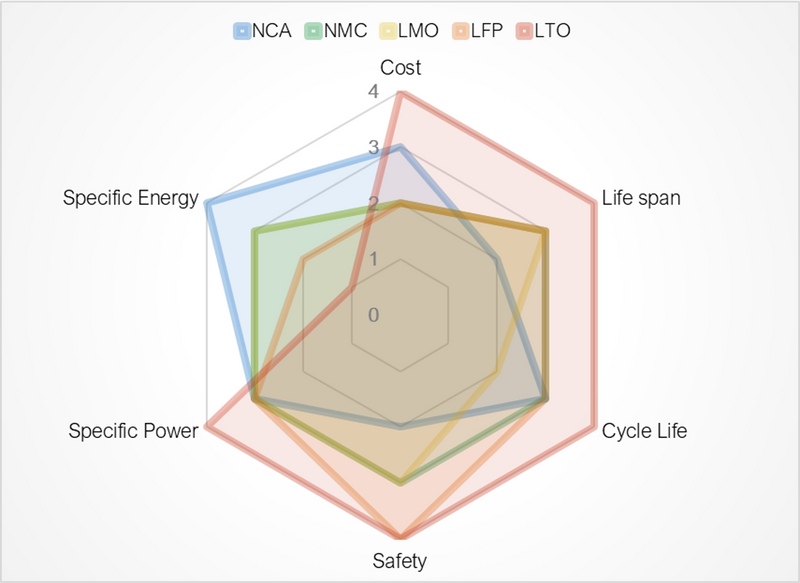
There are, however, several different chemistries of Lithium batteries on the market and are all suited to various applications. The lithium compound that Invicta uses is Lithium Iron Phosphate, which has a higher discharge/charge rate, cycle life, thermal stability and can cope with higher temperatures. But has a lower energy density and higher self-discharge rate compared to other lithium chemistries. This makes it an excellent option for electric vehicles and power tools and storing energy at power stations.
One of the other more common types of Lithium is lithium cobalt oxide (LiCo02), this type of Lithium is the type that is usually found in your phone, digital camera and laptops. Although this compound is the densest, its major drawback is its thermal instability which can create a fire. This is because the anodes can overheat, and the cathode produces oxygen during decomposition if not appropriately managed.
These are the two most common types of lithium battery on the market. However, there exist many more types of lithium combinations some of these include;
- Lithium Manganese Oxide (LiMn2O4)
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2 or NMC)
- Lithium Titanate
And with the industry continuing to explore different combinations for different applications, this list will continue to grow, each with its own unique advantages and disadvantages.
Lithium Cell Form Factors
A lithium cell can also come in three different types of constructions, each with its own benefits. The three types of lithium cells available are Cylindrical, Pouch and Prismatic.
The most common type of cell available is the Cylindrical which consists of sheet-like anodes, separators, and cathodes that are sandwiched, rolled up, and packed into a cylinder-shaped can. The round shape of the battery distributes the internal pressure from side reactions over the cell circumference almost evenly. However, this shape does not make efficient use of space, and the BMS can be more complex due to the high number of cells in applications such as EVs. Because they are the easiest and most cost-effective option to produce, they are the most common.
The Pouch design does not have a rigid enclosure and uses a sealed flexible foil that needs to be supported by an internal structure. It is a cost-effective option, and unlike the cylindrical cell, it can be stacked together to make use of the most space available. Because many cells can be stacked into a battery, it can be customised for applications that require a higher discharge rate or smaller physical size. A well-designed battery that uses a pouch cell should also make allowances for the lithium cell to swell 8%-10% and consider its susceptibility to high heat and humidity.
Finally, the Prismatic design, which Invicta utilises, consists of large sheets of anodes, cathodes, and separators sandwiched, rolled up, and pressed to fit into a metallic hard-plastic or aluminium housing in cubic form. This is a premium design that is the most mechanically stable, unlikely to swell and requires the least number of lithium cells due to its larger capacity and efficient use of space. Because it does have a smaller number of cells, this makes the matching of cell capacities much easier and requires a less complex BMS, further improving the longevity and performance of the battery and are generally designed specifically for deep cycle applications.
Lithium Cell Grade And Quality
A common misconception is that all lithium cells are of the same quality. In reality, Lithium is graded into three types. A grade, B grade and used, and depending on which grade is in your battery, will determine the longevity and contribute to the product’s final cost.
When a lithium cell does come off the production line, the cell’s capacity can be very different to the cell before it. Even the most precise and automated factories will still produce different capacity cells. The cells are then segregated based on how close they match with each other.
It is critical that when a battery is assembled, the cells match as closely together as possible. This is because a weak cell can cause the others to over-work during discharge. In charge, this weak cell can become overcharged because the stronger cells still require a charge. Eventually, this causes the weak cell to short. Overall, battery packs with well-matched cells will perform better and are proven to last longer than those with mismatched cells and will usually determine the warranty period for that battery. For example, a battery with 5-7 year warranty will consist of A-grade lithium cells that are very closely matched. Those with 2-3 years consist of B grade lithium cells that don’t match as well. Finally, batteries with one year warranty will consist of used or the lowest grade cell and are significantly mismatched.
Conclusion
Overall, when you are choosing a battery, ensuring it is fit-for-purpose should be your main objective. But understanding how they all differ, even chemistries of the same type, will ensure you know what you are buying and why it is priced the way it is.
Further Reading
https://www.science.org.au/curious/technology-future/batteries
https://www.science.org.au/curious/technology-future/lithium-ion-batteries
